In the ever-evolving landscape of healthcare, Cellbricks emerges as a pioneer in the regenerative medicine space, leveraging its advanced 3D bioprinting technology to create functional, patient-specific tissues. This innovative approach aims to dramatically shift the paradigms of organ transplant and tissue repair. Backed by a meticulous financial and strategic plan, Cellbricks recently closed a EUR 5 million Seed II funding round led by ACT Venture Partners, alongside co-investors like VU Venture Partners, Onsight Ventures, and b.value, underscoring our commitment to accelerating transformatively disruptive technologies.

Addressing the Regenerative Medicine Imperatives
Regenerative medicine confronts formidable challenges, primarily in developing functional tissues for clinical applications. The sector is marked by a severe mismatch between the demand for organ transplants and the available supply. A poignant example is the stark reality in the United States, where, in the first two decades of the 21st century, over 50,000 patients died every year while waiting for a liver transplant (Source). Additionally, the post-surgical mortality rate for those fortunate enough to receive a transplant can be as high as 16% due to various complications (Source). This scenario underscores a critical bottleneck within the field—a gap that Cellbricks is poised to bridge.
Cellbricks' advanced bioprinting technology stands as a beacon of innovation, set to transform the landscape of regenerative medicine. By enabling the fabrication of viable, patient-specific tissues, this technology not only aims to alleviate the transplant shortage but also enhances the quality of life and survival outcomes for patients globally.
Cellbricks: Revolutionizing Regenerative Medicine with Advanced Bioprinting
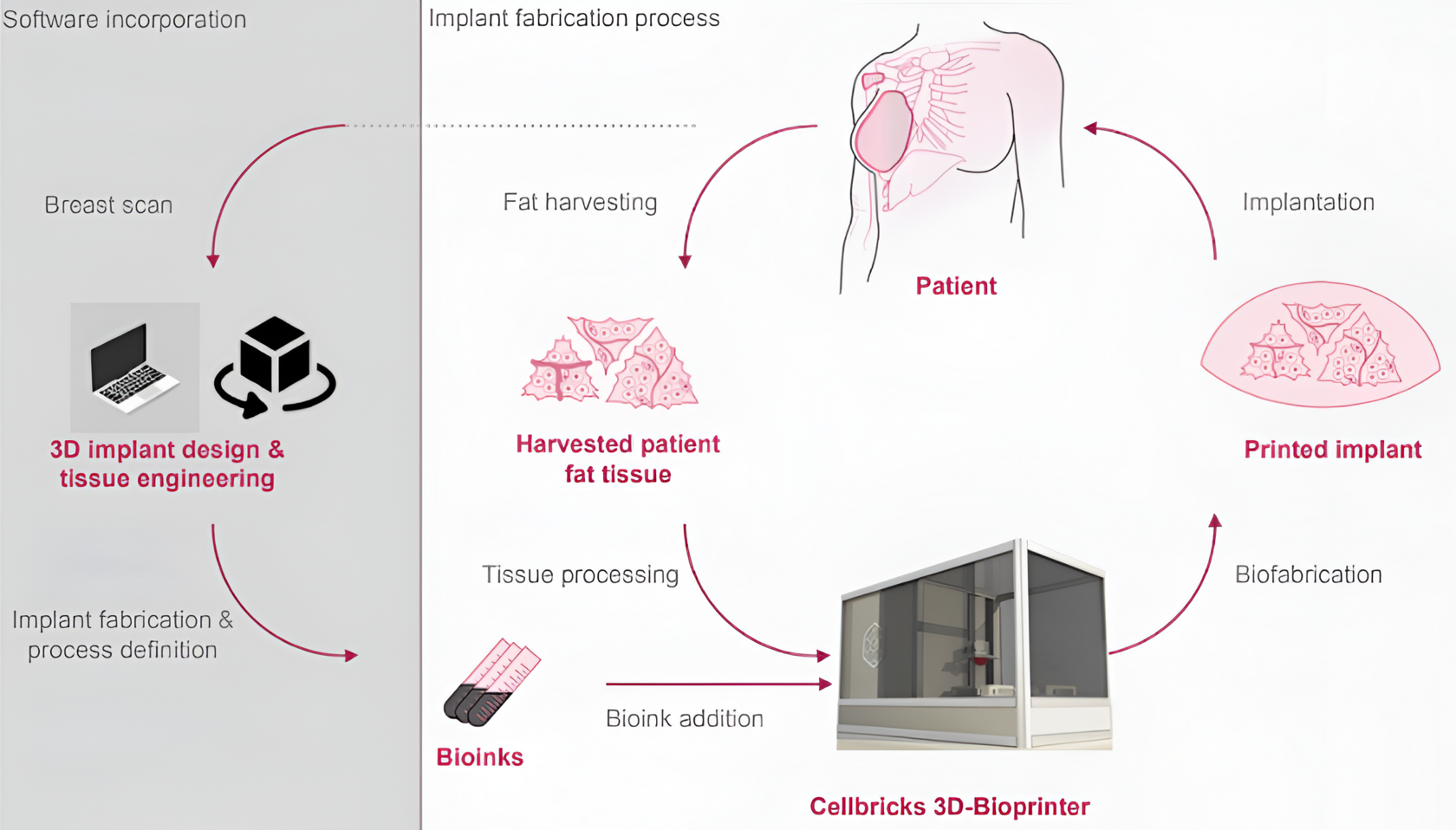
Cellbricks is at the cutting edge of regenerative medicine, leveraging its proprietary light-based biofabrication technology to transform healthcare outcomes. This platform transcends theoretical research by providing practical, scalable solutions for producing human tissues. By integrating their advanced 3D bioprinter into a Good Manufacturing Practice (GMP) compliant environment, Cellbricks consistently produces high-quality, reproducible tissues that adhere to stringent clinical standards.
Imagine a world where individuals like Nazli, a 36-year-old single mother of two, no longer face a grim prognosis due to liver failure. Thanks to Cellbricks' pioneering bioprinting technology, Nazli receives a bioprinted liver patch tailored from her own cells, significantly reducing her wait time and the risks associated with traditional transplants. This isn't just theoretical—it's a very real possibility with the advancements being made by Cellbricks.
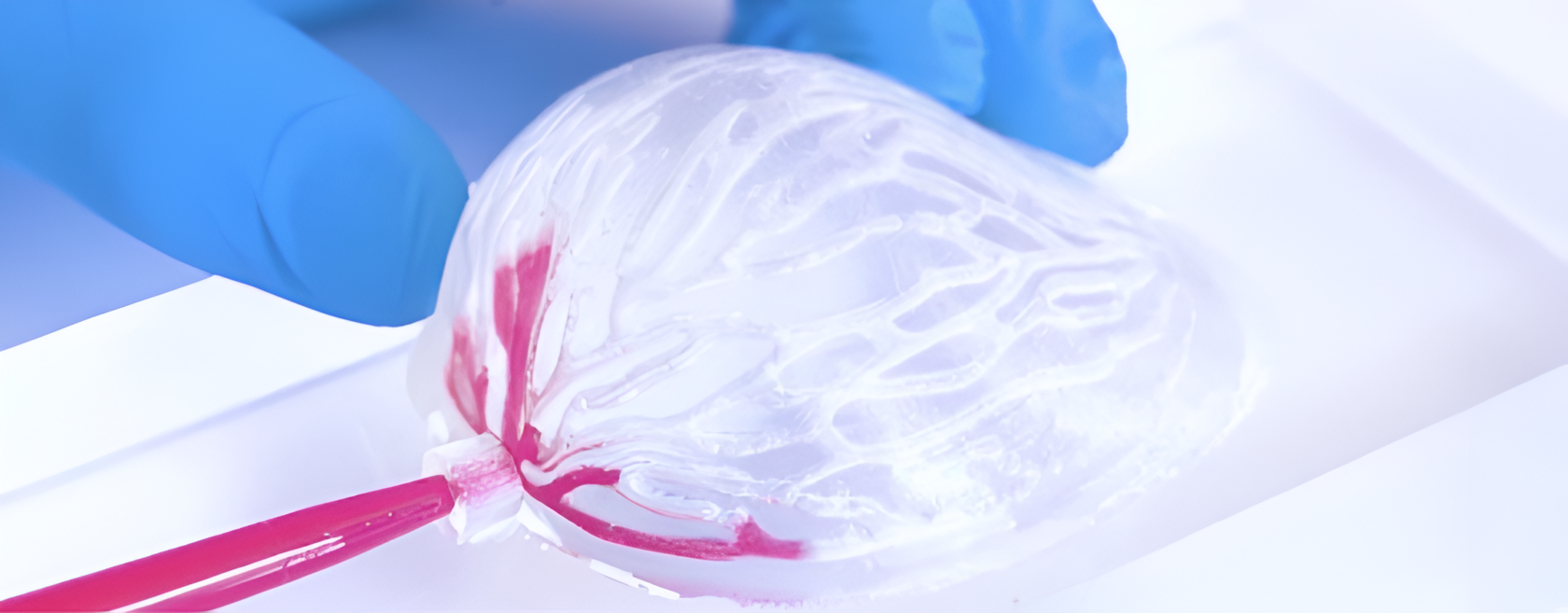
The broader societal implications are profound. Cellbricks’ technology could dramatically reduce the transplant waitlist, saving healthcare systems billions annually by decreasing the treatment costs associated with end-stage organ disease. Additionally, the ability to precisely control tissue architecture—including essential vascular structures—allows the creation of complex, multi-material tissues that mimic natural organ functions. This capability is crucial for developing treatments like liver patches and soft tissue implants, reducing dependence on scarce organ transplants, and enhancing the quality of life for thousands. By pioneering such advanced techniques, Cellbricks is setting new standards where organ shortages are no longer a barrier and treatments become safer and more effective. Their innovative efforts are poised to enhance patient outcomes and revolutionize the availability of life-saving medical interventions.
Pioneering Projects and Technological Advancements
Cellbricks is at the forefront of several groundbreaking projects that promise to revolutionize tissue engineering. With ongoing grants and subsidies supporting projects by Bayer G4A and Germany’s BMBF (Federal Ministry of Education and Research), Cellbricks is poised to make significant advancements in fabricating tissues such as liver patches and breast implants that could drastically reduce the need for traditional organ transplants.
Technologically, Cellbricks stands out with its multi-material DLP (Digital Light Processing) bioprinting capabilities, which enable the creation of complex tissue structures with a high degree of precision and scalability. This capability is complemented by the development of bio-inks that are devoid of animal-derived components, enhancing biocompatibility and safety.
Flagship Programs: Pioneering the Future of Medical Treatments
Cellbricks' flagship programs include:
- Liver Patch Implant: This human tissue implant with organ function is designed to support patients with liver dysfunction, particularly those suffering from pediatric rare diseases like Crigler-Najjar syndrome. Initially focused on these specific conditions, the liver patch will later be rolled out to address general indications of liver failure. Based on hepatocytes and featuring vascularization, this new delivery method for cell therapies can be implanted and integrated into compromised organs to restore function.
- Soft Tissue Implant: This vascularized human soft tissue implant serves various applications, including breast reconstruction following lumpectomy or mastectomy, as well as aesthetic procedures. These implants aim to fill tissue gaps in the human body, offering solutions for both medical and cosmetic needs. By providing natural, patient-specific alternatives, these implants improve outcomes and reduce complications associated with traditional methods.
Strategic Growth and Leadership at Cellbricks
Founded by Dr. Lutz Kloke, Cellbricks is steered by a leadership team of seasoned and visionary professionals. CEO Alexander Leutner and COO Dr. Simon MacKenzie bring a wealth of entrepreneurial and management experience from their successful ventures in biotech startups, such as Apodius. Their deep knowledge in biochemistry and medical technology, combined with the strategic and financial expertise of Joachim von Arnim, positions Cellbricks to ambitiously advance its innovative agenda.

The recent successful funding round marks a pivotal moment for Cellbricks, enabling the acceleration of key projects including in vivo studies and the integration of biofabrication technology into clinical settings. These initiatives are critical as Cellbricks advances the practical application of its groundbreaking bioprinting technologies.
Charting a New Era in Regenerative Medicine
The market for regenerative medicine is vast, with significant demand for solutions in areas such as breast reconstruction and liver transplantation, which account for USD 174 billion in market size. Cellbricks’ biofabrication technology addresses these needs by enabling the production of tissues that can be used in medical treatments, thereby reducing reliance on donor organs and improving patient outcomes.

Looking to the future, Cellbricks is preparing for global expansion, starting with the establishment of a strategic hub in Boston, Massachusetts. This new base will serve as a crucial connection to U.S. regulatory bodies and pharmaceutical leaders, fostering an environment that attracts top-tier talent and additional investment, and propelling Cellbricks toward its vision of global leadership in regenerative medicine.
Conclusion
ACT Venture Partners’ investment in Cellbricks is not merely financial but a strategic alignment with a vision that challenges the current limitations of medical science. Our partnership reflects a shared commitment to innovation, sustainability, and growth in the deep-tech industry. With its robust technology platform, talented team, and clear strategic direction, Cellbricks is well-positioned to lead the next wave of medical innovations, transforming lives and healthcare systems worldwide.
Join us in the journey to redefine the future of medicine. We invite you to learn more about Cellbricks' transformative work in 3D bioprinting at their website and to stay updated on their groundbreaking advancements by visiting our website and subscribing to our newsletter.

